Your location:Home >Automotive News >
Time:2022-06-15 12:34:29Source:
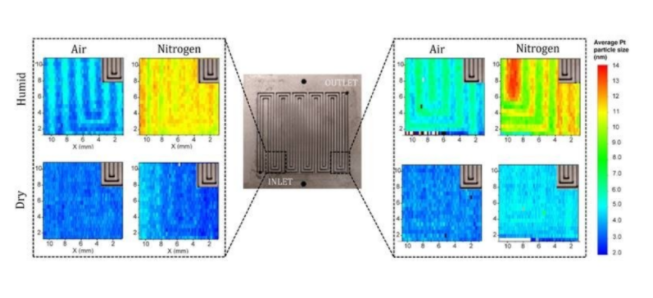
Fuel cells typically generate electricity from hydrogen, a "clean" fuel that produces only water when burned.Platinum is a key catalyst for this process.In fuel cells, however, platinum degrades unevenly.Replacing used fuel cells can result in the discarding of still usable platinum.According to foreign media reports, in order to improve the durability of fuel cells and reduce waste, the researchers explored the causes of non-uniform platinum degradation, including the humidity and gas environment (air and nitrogen) inside the fuel cell, and the geometry of the fuel cell flow channels. shape.
(Image credit: U.S. Department of Energy)
For automotive applications, polymer electrolyte fuel cells (PEFCs) are an attractive option due to their compact size, which can rapidly increase power output as needed.At the same time, the cost of this type of battery is lower and the durability is higher, so the application prospect is better.In light-duty vehicles, precious metal catalysts such as platinum account for about one-third of the cost of PEFCs.In this study, the researchers explored the degradation patterns of platinum catalysts in PEFCs.The obtained results help to develop simple and effective strategies to reduce the waste of expensive catalyst materials.
The team, led by the University of California, Irvine, and Bosch, studied commercially available catalyst-coated membranes that underwent accelerated stress testing.The researchers conducted tests in wet and dry conditions, as well as in air and nitrogen environments.Analysis of the samples involves a variety of methods, including scanning electron microscopy and several X-ray spectroscopic techniques.At the Advanced Light Source (ALS) at the DOE Office of Science User Facility, researchers used X-ray diffraction to map the size of aged platinum nanoparticles (the larger the particles, the more severe the degradation).In addition, X-ray computed tomography was used to image the aged catalyst layer.
The results show that platinum ages faster near the exit of the flow field, and in a nitrogen environment and higher humidity.Ageing is also faster at locations between the labyrinth-like channels that guide hydrogen through the catalyst layers.In the case of narrower flow field size, this effect does not exist.
The study concluded that adding countercurrent channels helps reduce inlet and outlet variability.Operating at higher temperatures will help reduce relative humidity.
Vehicles using PEFC are an excellent emission-free alternative to fossil fuel vehicles.The results from this research help to develop straightforward system-level strategies to increase durability and reduce cost to find lower-cost alternative catalyst materials for this type of technology.
Statement: the article only represents the views of the original author and does not represent the position of this website; If there is infringement or violation, you can directly feed back to this website, and we will modify or delete it.
Preferredproduct
Picture and textrecommendation

2022-06-15 12:34:29


2022-06-15 12:32:38

2022-06-15 12:32:01

2022-06-15 12:31:36
Hot spotsranking
Wonderfularticles

2022-06-15 12:31:09

2022-06-15 12:30:14

2022-06-15 12:29:33

2022-06-15 12:28:55


Popularrecommendations
